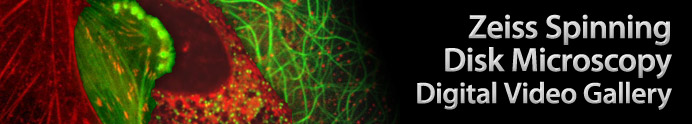
Monkey Kidney Cells with mEmerald-Tubulin and mCherry-Rab5a
Each of the 13 protofilaments that compose a microtubule consists of alpha-tubulin and beta-tubulin molecules are organized in an alternating pattern. When they come together to form a new microtubule, new bonds are formed among the tubulin heterodimers. Between the alpha-tubulin molecules and adjacent beta-tubulin molecules along the longitudinal axis, interfaces with significant binding energy are formed. Additionally, lateral interfaces occur between protofilaments that edge one another, creating bonds between tubulin monomers of the same selection because of how the protofilaments align with one another (alpha-alpha, beta-beta).
Endosomes are a type of membrane-bound vesicle produced through a complex series of processes together referred to as endocytosis. Endocytosis essentially involves the invagination of a cell's plasma membrane in order to surround macromolecules or other matter, encircling the material, and then budding a vesicle into the surrounding cytoplasm. After vesicles are released into the cytoplasm, the activities in which they are involved vary depending on their contents.
The digital video sequence presented in this section explores endosome dynamics in relation to the microtubule network. Endosomes were visualized with mCherry fused to Rab5a, an early endosomal marker. mCherry was one of the original six monomeric fluorescent proteins exhibiting emission maxima ranging from 540 nm to 610 nm produced by Shaner and colleagues through the directed evolution of mRFP1. The microtubule network was tagged with a chimera of mEmerald and tubulin.