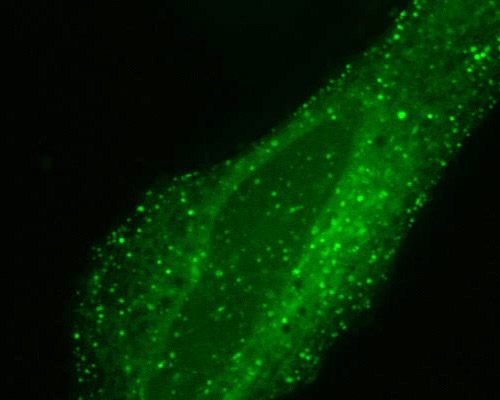
Human Cervix Adenocarcinoma Cells with mTag-GFP2 and Rab5a
The cell surface receptors CCR2 and CCR4 bind CCL2. CCL2 displays chemotactic activity for basophils and monocytes, but not for eosinophils or neutrophils. If the N-terminal residue is deleted, CCL2 is converted from an activator of basophils to a chemoattractant of eosinophil. CCL2 causes degranulation of basophils and mast cells, a result enhanced by previous treatment with IL-3 and other cytokines. It supplements monocyte anti-tumor activity and is necessary for formation of granuloma.
Rabs are a family of Ras related proteins that chiefly are involved in the cell with vesicle trafficking and signaling. The first Rab proteins were identified in the mid-1980s. Now more than 70 Rab proteins have been found in human cells alone. Studies indicate that the small proteins and their functions in vesicle trafficking are rather complex. In microscopy applications, several of the Rab proteins are useful as markers of endosomes in various stages within the cell.
Early endosomes were fluorescently tagged in cultured human cervix adenocarcinoma cells (HeLa line) with mTagGFP2 fused to Rab5a. mTagGFP2 is a green fluorescent protein developed via mutation of the Aequorea macrodactyla GFP-like fluorescent protein. mTagGFP2 exhibits brightness similar to that of GFP, but is considerably more photostable. Peak excitation and emission of mTagGFP2 occur at 483 and 506 nanometers, respectively.