Among the most useful FRET applications in cell biology is a technique that involves the fusion of two fluorescent proteins to the ends of an environmentally sensitive protein or peptide to act as a biosensor of specific cellular functions. Fluorescent protein biosensors have found widespread utility in reporting on a diverse array of intracellular processes. By creatively fusing FRET-capable pairs of fluorescent proteins to biopolymers that perform critical functions involved in various aspects of physiological signaling, research scientists have developed a host of new molecular probes that are useful for optical live-cell imaging of important processes such as calcium wave induction, cyclic nucleotide messenger effects, pH, membrane potential fluctuations, phosphorylation, and intracellular protease action.
The tutorial initializes with an image of the specimen displayed in the Ratio Color Image window and the corresponding spectral profile shown in the Spectral Analysis window. The tutorial automatically starts in the Auto Play mode with the YC3.60 cameleon biosensor being stimulated by the addition of histamine, which invokes a FRET response. The time plot in the lower portion of the window maps the reaction of the biosensor to the exogenous stimulant. At any point, the tutorial can be halted by clicking on the Stop button. In this mode, the Time slider can be used to transition to any time point. The Choose A Biosensor pull-down menu can be used to select between the cameleon and a caspase biosensor.
In FRET applications, spectral imaging can be considered a variation of the sensitized emission technique that relies on excitation of the donor alone, followed by acquisition of the entire emission spectrum of both the donor and acceptor fluorescence instead of capturing data in two independent channels. Until the introduction of laser scanning confocal microscopes designed for spectral imaging, the technique was largely limited to spectroscopy experiments using cuvettes and purified fluorophores. Spectral imaging FRET assumes that gathering of the entire fluorescence spectrum will enable overlapping spectral profiles to be separated according to the distinct shapes of the spectra rather than simply monitoring emission intensity in a limited bandwidth region using a filter. Thus, by collecting the entire spectrum from both the donor and acceptor (see Figure 1(a)), it is possible with spectral imaging to determine the levels of donor and acceptor fluorescence, and from that information (combined with controls), to calculate the FRET efficiency.
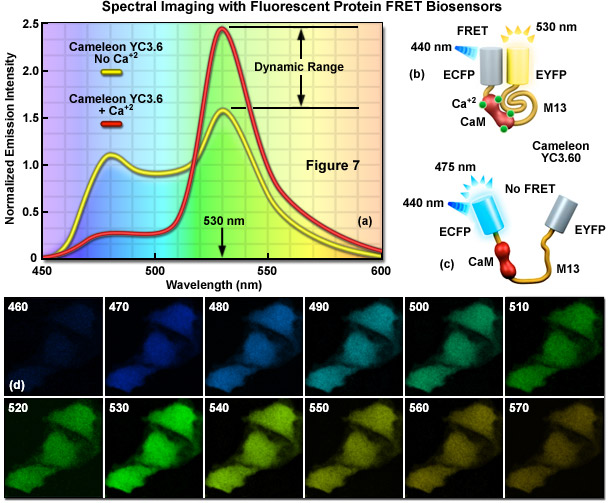
Presented in Figure 1 is a ratiometric spectral imaging measurement for the fluorescent protein calcium biosensor known as cameleon YC3.60, as described above. The spectral profiles of YC3.60 in the presence (red curve; Figure 1(a)) and absence (yellow curve) of calcium demonstrate the high dynamic range of the probe at 530 nanometers. Cartoon drawings of the cameleon biosensor are presented in the presence (Figure 1(b)) and absence (Figure 1(c)) of calcium to illustrate how the alkali metal induces a conformational change in M13 to relocate the two fluorescent protein moieties closer together. A lambda stack of images in 10-nanometer intervals of cells expressing the cameleon in the presence of high calcium concentration are shown in Figure 1(d). Note that the highest intensities are observed for the lambda plane containing emission wavelengths from 530 to 540 nanometers.
Contributing Authors
Adam M. Rainey and Michael W. Davidson - National High Magnetic Field Laboratory, 1800 East Paul Dirac Dr., The Florida State University, Tallahassee, Florida, 32310.




